Moving Away from Animal Models
I’ve spoken with scientists and life science entrepreneurs for years. The shortcomings of animal model research stuck out like a sore thumb. Despite the discoveries that mice have helped us make, there’s always something we can’t replicate from them. We’re also not returning the favour with the way we’re treating our animals.
That’s where organ-on-a-chip models come in. These tools can mimic complex biological processes by combining the ingenuity of engineering with the intricacies of biology. Imagine a device made of tubes and pumps. They would represent blood vessels while the pumps push the blood along. Then you could have a chamber filled with cells from an organ you’re interested in studying. Lastly, shrink the model down and track the cells with a microscope or another lab device. Now, you have a basic organ-on-a-chip model.
You can track how cells respond to multiple drugs at the same time. You can also reduce sample usage and incubate your cells within your model. And you can tweak the growth conditions to replicate a patient’s clinical status. Imagine the cost reductions and easing of your research now that the FDA no longer requires animal tests before human drug trials.
Now that you’ve pictured the possibilities, let me introduce you to Boyang Zhang. Boyang is the CEO and founder of Organo Biotech. After starting out with lung fibrosis research, his startup now develops organ-on-a-chip models that help researchers answer their most pressing biological questions. Those traits made him the perfect person to interview about organ-on-a-chip models.
So, for our next edition of the Innovation AveNEW 2025 series, we’re interviewing a professor from my alma mater, McMaster University. Read on to learn about organ-on-a-chip models and see how you can benefit from their services as you attend SLAS 2025!
The Interview
About Organ-on-a-chips
PN: Let’s begin with the problem you’re addressing at OrganoBiotech. You mention in your website that traditional preclinical models are no longer adequate for studying complex chronic disease. Why is that?
BZ: If you’ve heard that a pig heart is like a human heart’s, that’s not an urban tale. Although many differences to arise, many similarities also exist. Hence, when we speak of traditional preclinical models, we refer to animal models like pigs. Rodents, fish, and even more complex mammals are just a few examples of such models. As we’ve seen with the pig heart, scientists use animal models because they mimic several aspects of human physiology. However, some aspects of human physiology fail to be replicated in animals. With my line of work, animal models do well in replicating short-term fibrosis, but fail to represent the chronic, progressive disease observed in many fibrosis patients.
PN: Knowing the limitations of animal models, you turned to organ-on-a-chip models to model lung fibrosis. What are they, and how can they improve upon existing disease models?
BZ: Organ-on-a-chip models mimic human tissues by disassembling their complex structure and rebuilding them step-by-step. I noticed that interviewed someone who is pitching a LEGO DNA set on LEGO Ideas. Think of that but instead building an organ up from its constituent cells and tissues. The model doesn’t have to be completely organic. In fact, organ-on-a-chip models regularly feature tubes and pumps to simulate the movement of bodily fluids across the organ. With it, one can introduce immune system modulators and other compounds to test various aspects of human physiology.
Organ-on-a-chip models allow researchers to study interactions between specific cell types and understand fundamental biological processes. With organ-on-a-chip models, we can create snapshots of airway function at various stages of pulmonary fibrosis. This is especially useful since we know that sustained lung damage and immune responses distinguish late-stage and early-stage fibrosis. Now, while the ultimate goal is to create models that are fully predictive of human physiology, the journey of refining these tools yields valuable insights. These systems can offer more physiologically relevant data than traditional models and reduce costs associated with late-stage drug development failures. Importantly, they align with the push toward alternatives to animal models.
PN: What did you find was the most challenging physiological process to replicate?
BZ: I believe no human process is as complex as our immune systems. It comprises many cell types, cytokines, and other molecules that affect every organ in our bodies. Just replicating one aspect of the immune system can encompass the lifetimes of multiple research groups. Organ-on-a-chip models grant us a level of control over this intricate system. We can systematically add or remove specific cell types and modulate the concentrations of various mediators and antigens. In turn, organ-on-a-chip models can help us gain further in-depth insights into our immune systems that animal models cannot.
PN: As you hold many years of experience working with organ-on-a-chip models, what’s your advice for scientists who strive to model similar processes?
BZ: For anyone working with organ-on-a-chip models, I say don’t fear the complexity. Embrace it. Tackle the immune system or any other system one step at a time. Focus on stepwise improvements and let your research questions guide you. Lastly, make sure you leverage tools that help you dissect cellular interactions precisely and iteratively.
About the OrganoPlatform
PN: As you worked on organ-on-a-chip models, your iterative improvements led to producing the OrganoPlatform. Tell us about what it is and how you anticipate it will aid researchers today.
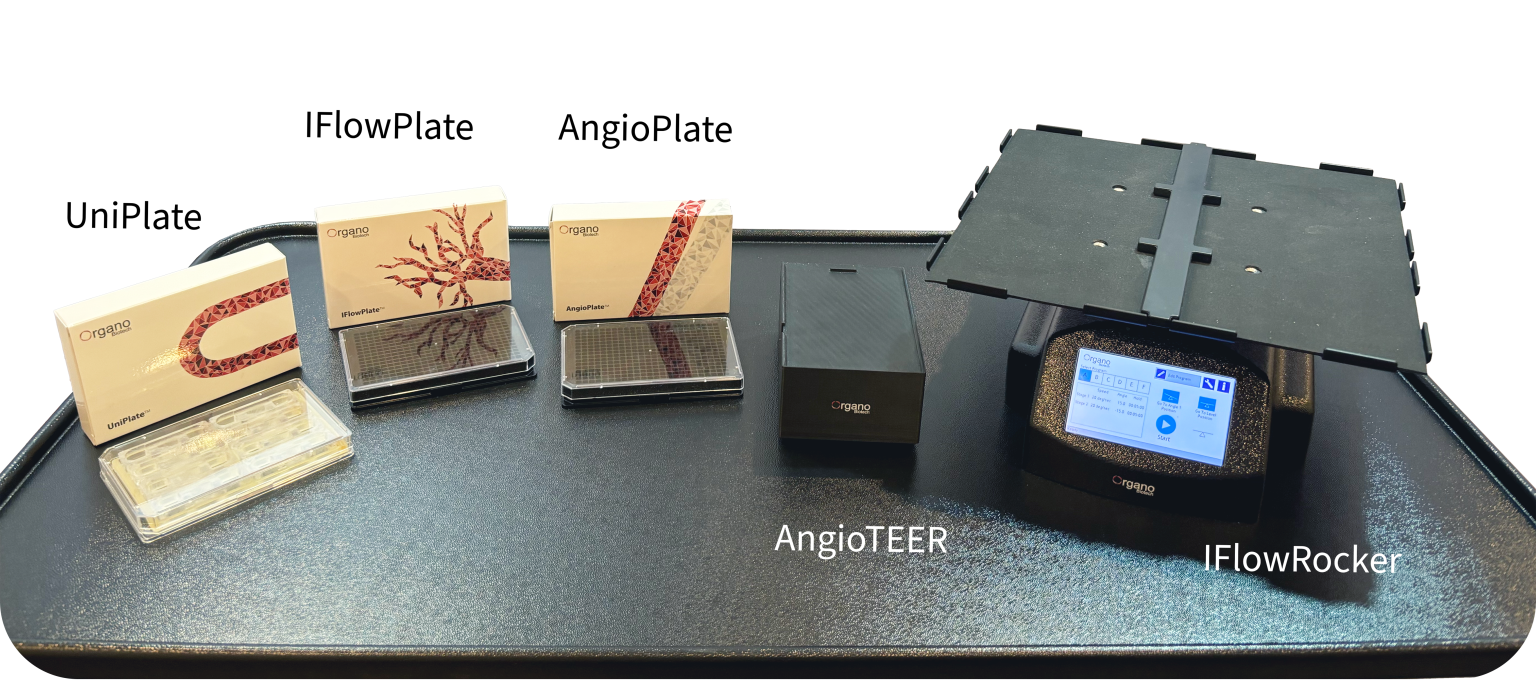
BZ: OrganoPlatform is our way of compartmentalizing tissue models. The platform comprises multiple 384-well and 24-well plates that miniaturize cellular processes in each of the wells. With it, scientists can create organ-on-a-chip models of varying complexity that meets their experimental needs. Our platform also does not work alone. We also provide supporting equipment, such as IFLowRocker, AngioTEER, and OrganoPulse that mimics tissue perfusion, enables barrier measurements, and models mechanical stimulation. The best part about our platform is that it’s automated, reproducible, and high throughput. Our plates are fully compatible with existing and novel robotics systems, reproduces tissue dynamics, and helps scientists study multiple conditions at once.
PN: You must have acquired a wide array of experts to help you produce the OrganoPlatform. What about McMaster University and Canada’s know-how enable you to develop it?
BZ: The biotech ecosystem is different from academia. When we conduct research, we’re homing in on real-world impact. Successful biotech companies go beyond just publishing academic papers and address pressing scientific and clinical needs directly. Years of collaborative research by talented students were crucial for getting the system off the ground. On top of that, McMaster’s Innovation Park and other facilities create an entrepreneurial ecosystem and a robust support network. Such a network helped us receive government funding and transition our technology from the lab to our end-users.
PN: You mention transitioning away from academic research and into meeting pressing needs. What are your current plans to commercialize your organ-on-a-chip and your CRO services? What challenges have you faced in advocating greater adoption of such systems in scientific research?
BZ: As a new technology, the adoption process can be slow and requires ongoing collaboration with end-users to refine our systems. Even so, I have seen a lot of hype and genuine potential surrounding organ-on-a-chips. As regulatory and industry trends shift toward non-animal alternatives, achieving widespread adoption of organ-on-a-chips will require patience, education, and close partnerships with diverse stakeholders. From McMaster University, we remain ever committed to facilitating this transition by continuously improving and demonstrating the value of the OrganoPlatform.
PN: In line with your commitment, congratulations on being selected as an Innovation AveNEW company. How will accessing SLAS’s resources through this award help you scale up your company?
BZ: This is our first time attending the SLAS exhibition and conference, and we are excited about the opportunity to engage with this vibrant community. It’s a chance to showcase our work, learn from industry leaders, and build new connections. Personally, I’m also thrilled to escape the Canadian winter and visit San Diego for the first time!
Author
-

Paul Naphtali is a seasoned online marketing consultant. He brings to the table three years of online marketing and copywriting experience within the life sciences industry. His MSc and PhD experience also provides him with the acumen to understand complex literature and translate it to any audience. This way, he can fulfill his passion for sharing the beauty of biomedical research and inspiring action from his readers.
View all posts