Guiding cells with foreign cargo
How can we get cells to do what we want when our bodies are sick? We change how they behave by introducing biomolecules into them.
It may be just one strategy we can use, but it can be an effective one. By directly injecting cells with therapeutics or other molecules, we can alter how they behave in our bodies. We can enhance their ability to kill cancer cells. We can tell them to produce biomolecules for us. We can even test drugs in target cells directly.
However, we’ve mostly done it on cells that have gotten used to testing in the lab. Our bodies’ cells haven’t. Most efforts to introduce foreign material into them either kill the cells or fail to induce the desired outcomes.
So, how do we resolve this issue?
That’s a question that Armon Sharei and his teams at Portal Biotechnologies are tackling. They developed a gentle approach to introduce cargo that is reproducible and safe. And it manifested into the Portal Galaxy, a product they finished developing just in time for SLAS 2025. With the Portal Galaxy, you just need to give a gentle squeeze your primary cells, and you can introduce molecules of many sizes, from DNA to proteins.
Read on to hear more about this solution.
The Interview
Cargo Loading into Cells: an Introduction
PN: Let’s start by talking about introducing foreign material into human primary cells. What are primary cells, and why would someone want to do this?
AS: Primary cells are any kind of cell taken directly from living tissue. They’re different from cell lines, since they’re not genetically modified to grow perpetually in the lab. Even so, there are many reasons for introducing foreign cargo into cells. Altering cellular behaviour before being reintroduced into the body stands as the biggest reason for this change. Let’s consider some of the cargo you can introduce into the cells:
- Genetic material: Introducing DNA and RNA into cells has been a staple of gene editing. DNA and RNA would enter through permeable cell membranes so that the cells would either integrate the DNA into their genomes or translate the foreign RNA into functional proteins.
- Proteins: Proteins are substantially larger than nucleic acids. But they too can be introduced into cells. With this, researchers can chemically modify a protein or assemble a complex in different ways before delivery. Doing so can test protein function and more precisely manipulate how a cell behaves.
PN: How do we currently introduce foreign genetic material into cells, and what’s the biggest shortcomings with the methods we use now?
AS: So, there’s three ways we can introduce foreign cargo into cells right now. We can walk through them below:
- Electroporation: Electroporation stands as the most common way to introduce foreign material into cells. As the name suggests, you electrocute the cells, creating small pores that allows foreign cargo to flow into the cells. However, cells hate getting electrocuted. The cells that can handle electrocution the most are resilient cell types programmed to multiply in the lab and withstand stress. In contrast, most normal cells would die if they were exposed to such a stressful condition.
- Nanoparticles: Nanoparticles also transport foreign materials to enter cells, but with the help of engineering. They can compose naturally occurring molecules like lipids in lipid-based nanoparticles. They can also be made from synthetic polymers that modulate the cell’s likelihood of taking the cargo into the cell. However, nanoparticles have only been tested on certain cell lines. The synthetic polymers on nanoparticles can also trigger unwanted reactions.
- Viruses: The viruses that infect us aren’t the only kinds that exist. Additionally, we can engineer them in many ways so they can be safe for human use. In the latter situation, we can load viruses with cargo that they inject into cells. Virus loading requires further testing, in part because the kinds of cargo that fit into viruses are limited at best.
The story behind Portal Biotechnologies
PN: With these weaknesses, I’d expect that you sought an easier, more effective way to deliver cargo into cells. Was that how you founded Portal?
AS: Partly. We discovered our current technology by accident. None of the methods we tested could put cargo into cells without killing them. We even had a gun that we would fire molecular compounds into cells. The problem was that none of the methods we tried previously worked. Either we saw no penetration, or the cells were getting killed afterwards. Then, when we go to squishing cells at high speeds, we suddenly discovered that gentle pressure did exactly what we wanted. Squishing the cells disrupted their membranes, opening pores large enough for anything to enter. Chemistry and charge didn’t matter either, which meant we removed a ton of variables that affected cargo entry.
PN: Give us a look at the technical side of how your technology works. How large are the pore sizes you make, for instance?
AS: You can check out our picture below to see how our technology works (Figure 1). Imagine the cells are floating within a cartridge. Our pressurizer introduces flow into the cells, which would go through a thin piece of silicon with specific pore sizes. When the pores are smaller than the cell, that creates the holes in the cell membranes that allow foreign material to be added into the cells. With this method, we’ve used 3- to 15-micron sized pores to create pores 100 nanometres in size. That pore size allows any kind of biomolecule to enter cells. RNA, DNA, protein, even viruses.
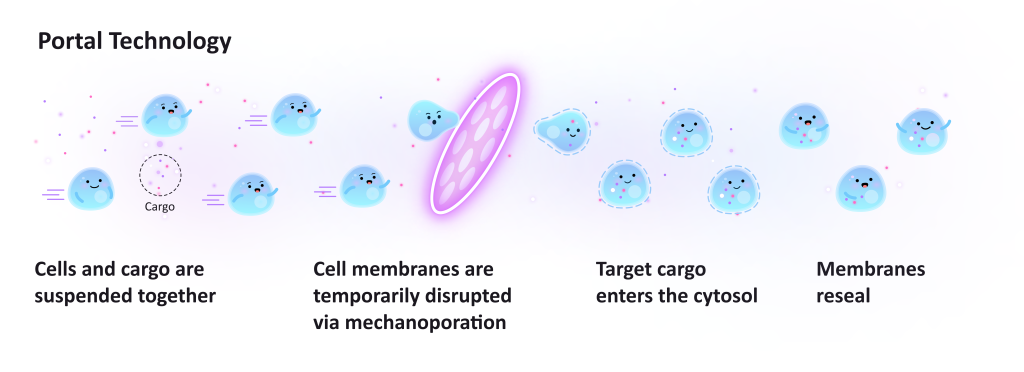
PN: Are there other variables one must consider when creating pores with Portal’s products?
AS: How fast the cells go through the pores is the only other variable scientists need to consider perturbing them with our products. The faster they enter, the harsher the protocol will be. In turn, the sizes of the pores will be larger so more material can enter the cells. Regardless of the protocol, the cells must be transferred into a solution containing the cargo you want to load within 30 seconds. The cell holes will close gradually, within 30 seconds of perturbation.
PN: It’s amazing that you could make this process so much easier just by squishing the cells gently. You even fine-tuned it to be consistent! Turns out all it took as to be nice to the cells.
AS: It surprised us all too. Nonetheless, it’s good that we made the protocols easy for the cells to handle. Our company focuses on patient cells because of the many ways we can use them to enhance patient care. We can engineer cells to go after specific diseases like cancer by introducing material that tells them to attack structural features unique to cancer cells. You can also program stem cells to become a neuron to mitigate Parkinson’s Disease by introducing content that tells them to become neurons.
The thing about patient cells, however, is that they’re not programmed to take big risks. If they think something’s wrong with them, they will undergo programmed cell death, or apoptosis. It’s their self-defence mechanism against developing cancer or an infection. Conversely, we typically work with cell lines that can grow indefinitely. They’re more used to extreme situations like being electrocuted.
Entering the World of Automation at SLAS 2025
PN: You recently shared word of your latest product, the Portal Galaxy. With automation being a prominent theme in SLAS 2025, how would the Galaxy facilitate high-throughput cell manipulation and automation?
AS: Electroporation and other techniques normally require the reagents, cells, and cargo to be mixed within the same vessel. With the Portal Galaxy, you won’t have to do any of that. Instead, we run the cells through our machine without any cargo present. Then, the cells enter a trough where a liquid handler can grab the cells and dispense them into a plate while their membranes are still open. Our setup allows those disrupted cells to be dispensed quickly enough for the cargo to enter the cells.
We already have several companies who are buying into what we’ve built. At SLAS 2025, Eppendorf became a launch partner of the Portal Galaxy (Figure 2). There, you can see how we apply robotics to make introducing foreign cargo into cells easier and reproducible. Moreover, the best part about the Galaxy is that we made it fully compatible with existing automation tools such as liquid handlers. Its ease of use and consistency will make cell therapies and many other disciplines that much more affordable and easier to do across the life sciences.

PN: Congratulations on winning the SLAS New Product Award, a clearly well-earned achievement! How do you feel about winning the award and how will it help you scale up your solution for your stakeholders?
AS: I and the rest of our team at Portal are especially happy that we won the award. We hunkered down to get the Portal Galaxy ready for SLAS. Plus, this year was the first time I attended a SLAS conference. My background is much more cell therapy oriented than automation and drug screening. Nonetheless, we’re delighted to receive recognition for our product launch given the effort we put towards launching the Galaxy on time for SLAS.
Going forward, the press coverage and validation of our products will help make potential customers feel excited to try our products. We’re constantly looking for new life science and business partners to integrate with and work together. At SLAS 2025, we discovered the rise of drug screening and how new tools are making high throughput screening possible. You see, many of the drugs being screened are too large to enter cells. That helped us open a new application for our Portal Galaxy. We can make pores large enough to fit even these larger drugs, such as heterobifunctionals or peptide-based drugs.
All in all, SLAS 2025 was such an eye-opening experience. I’m grateful that SLAS awarded us the New Product Award. And I look forward to helping more clients across the drug development space with the Portal Galaxy.
Author
-

Paul Naphtali is a seasoned online marketing consultant. He brings to the table three years of online marketing and copywriting experience within the life sciences industry. His MSc and PhD experience also provides him with the acumen to understand complex literature and translate it to any audience. This way, he can fulfill his passion for sharing the beauty of biomedical research and inspiring action from his readers.
View all posts




