Why consider solubility in drug development?
Drug development may seem simple enough. You’re testing a compound for its ability to treat disease without side effects. Yet, this singular goal consumes billions of dollars and countless hours of manpower. Worse still, you can’t guarantee that a compound will act as you think it will. Many variables, from how large it is to how it’s administered, can affect how the human body reacts to any drug. Among these variables, drug solubility stands as one of the most vital.
How well a drug dissolves in water determines whether our bodies will absorb it. If a drug is too insoluble, our bodies will excrete the drug without ever reaching the tissues we’re trying to treat. Tests that analyze a drug’s solubility in water will hence be essential to ensure that the drug doesn’t fail clinical trials before they even start.
We discover thousands of new drug candidates each day. And we can’t screen them all without a high-throughput method to test their solubility and other parameters.
So, how can we monitor the solubility of all these drugs?
Nathan Dupertuis, Co-founder of ORYL Photonics, took notice of this problem. In response, he and his team developed an in-house technology that measures drug solubility at high-throughput rates. Called the ORYL F1, his instrument has become the first to offer rapid and sensitive solubility testing at high-throughput rates. And in today’s interview, you can learn how you can use the ORYL F1 to determine whether your drug is soluble enough for clinical trials.
Read on to learn how solubility affects drug activity, how the ORYL F1 works, and how you can integrate it into your drug development pipelines.
The interview
Measuring drug solubility: an introduction
PN: Let’s start with the basics of solubility since your technology is directly connected with it. What determines how soluble a compound, whether a drug or something else, is within a solvent?
ND: Solubility refers to how well a substance, the solute, dissolves within a solvent. At the molecular level, a wide range of chemical interactions determine whether a compound can dissolve within a solvent. These can include stronger bonds such as hydrogen bonds, or weaker interactions such as van der Waals forces.
With that said, several factors will affect how these interactions in solutions arise and how soluble a solute is within a solvent is:
- Polarity of your solvent and solute: You may know the common saying that goes, “like attracts like”. With solubility measurements, polar solutes dissolve in polar solvents, and vice versa.
- pH: pH refers to how acidic or basic a chemical is. Generally, acidic salts are more soluble in basic solutions and basic salts are more solute in acidic solutions. That’s because the hydrogen protons from acidic solutes remove hydroxide from the basic solvent. The same works vice versa, where basic anions react with excess hydrogen ions from acidic solvents, increasing solubility.
- Temperature: Changing the temperature of the solution can increase or decrease the solubility of a compound if the reaction that dissolves a solvent releases energy or takes it respectively.
All these variables are but only a fraction of the factors to consider when developing a drug that’s soluble within our bodies. For example, if we’re dealing with a low-soluble drug, we can include agents, compounds that can help the active ingredients become more soluble. We call these compounds excipients, and you’ll need to test which excipients will solubilize your drug candidates the best.
PN: I imagine that we know all these things about solubility thanks to knowing how to measure the solubility of a solute in a solvent. How do we currently measure solubility?
ND: There are at least two major classes of technologies for measuring solubility. The first of these are optical technologies. One example is nephelometry, which is well-established for various applications, including drug testing. If you’ve tested whether a water source was potable, you’ve probably used a nephelometer. Nephelometers pass a beam of light through a sample and measure how much the light is attenuated. The less light goes through, the more turbid the solution is. In solubility measurements, a sudden rise in turbidity means that the solution is no longer soluble.
Chromatography systems, often coupled with mass spectrometry, comprise the other class and have seen more recent adoption. Here, you would prepare a saturated solution of the solute (drug) in a solvent, and filter it to remove the undissolved excess of solute. Then, you would prepare the solution for mass spectrometry. You can then use the intensity of the signal peak to determine the saturated concentration of the solute, called the solubility limit.
The shortcomings of current solubility testing methods
PN: With at least two methods to measure solubility, why would you want to develop an alternate approach to measuring solubility?
ND: Although both approaches have their place in the life sciences, both hold weaknesses that complicate solubility measurements at high throughput rates. Nephelometry is cheap and easy to implement. However, the resolution of nephelometry may not differ from examining solubility with the naked eye. That level of sensitivity isn’t good enough when minute differences in solubility can drastically reduce drug absorption.
On the other hand, chromatography and mass spectrometry improves on sensitivity substantially. However, mass spectrometers are much more expensive and require far more hands-on time to prepare and run the samples. In the process, you can burn through a lot of a candidate compound. Performing extensive profiling of different conditions or conducting temporal dynamics studies would then require high quantities of compound which is often scarce and expensive at this stage.
Both methods also fail to observe instances of aggregation within the solution. Firstly, certain types of molecules, most notably proteins, can be prone to aggregation. Aggregation at this scale increases the likelihood of aberrant immune responses and hides active sites. These, in turn, reduce the clinical effectiveness of a drug. Additionally, aggregation reduces drug yields in cell-based production methods. That leads to enormous losses of compound and chemicals in production. The worst part of all this is that aggregation can occur even when the formulation appears soluble to the naked eye.
PN: Despite the shortcomings, it’s clear that solubility testing is still important to conduct.
ND: Absolutely. the drawbacks that come with current methods of testing shouldn’t stop us from measuring solubility. Ignoring solubility and aggregation studies early on creates even more costs from modified iterations and lots of back and forth later within a drug development pipeline.
Introducing the ORYL F1
PN: You developed your company’s flagship technology, the ORYL F1, to address the technological challenges head on, especially aggregation.. How does the ORYL F1 do this?
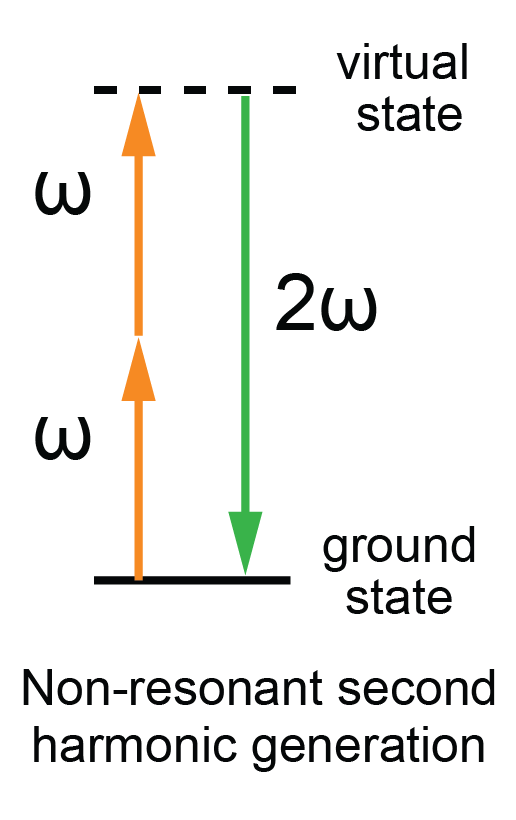
ND: The ORYL F1 uses a light scattering technology called Second Harmonic Scattering (SHS) (Figure 1). In SHS, infrared light is shined onto a medium so that the dissolved material will re-emit light at twice the frequency of the incident light. This light is known as second harmonic light. However, unlike fluorescence, SHS does not transfer any energy to the molecule, nor does it heat up the solution. That means we can measure solubility without allowing temperature to affect such measurements.
With SHS, the greater the scattering, the less soluble a solution is, indicted by increased intensity. With this conversion from infrared to green light, SHS is very sensitive to the structure of the samples and can detect aggregation events even before turbidity sets in.
In addition, unlike fluorescence, the kind of SHS we use does not transfer any energy to the molecule, nor does it heat up the solution. That means we can measure solubility without allowing temperature to affect such measurements. And as it is non-destructive, we can measure kinetics, which is very useful, for instance, for supersaturation or reversibility studies. Alternatively, we can keep the sample to measure it afterwards with orthogonal technologies.
Another advantage of this optical process is that it does not suffer from multiple scattering in very concentrated samples. This means we can measure High Concentration Liquid Formulations (HCLF) at in-vial concentration up to 400 mg/mL, only by shining a bit of light through.
PN: That sounds extremely promising if you’re measuring solubility and wishing to save precious material. Do you have standards and controls to standardize aggregation measurements with SHS?
ND: We have prepared at least two controls to affirm our SHS measurements: caffeine and albendazole. The former is a highly soluble molecule, while the latter has a well-known solubility limit. We’re also looking into other standards and controls to further delineate and understand the dynamics of SHS and solubility.
Integrating automation into solubility measurements
PN: Now as you know, SLAS 2025 is all about automation. How do you ensure that the ORYL F1 protocol can be automated?
ND: With automation taking centre stage in many aspects of life sciences research, we knew we had to make ORYL F1 protocols easy to automate. First off, we adapted the protocol for full compatibility with 96 and 384-well plates. We prepare dilution series with several replicates of a set of compounds of interest and of the controls in the well-plate. hat means we can test for several compounds on the same plate and screen SHS very quickly. As a reference, we can measure solubility in 15 minutes for a single 384-well plate with the ORYL F1. Once the screening is complete, our automated software processes the data and generates the solubility values in a straightforward way. That’s a huge improvement in throughput compared with existing technologies. Furthermore, our plates are fully compatible with automation robots that can perform dilution series quickly such as the Echo acoustic dispensers. Overall, this automated platform combines high sensitivity and high-throughput traits with very low compound consumption. That’s the kind of combination that fills the gap in current technological needs from researchers and provides them with the foresight they need for preclinical research.
PN: It sounds like you have an exciting solution for measuring solubility quickly and accurately, and it’s culminated in winning the SLAS Ignite Award. Congratulations on winning! How will it help you launch the ORYL F1 this year?
ND: As a startup, we’re seeking attention from diverse stakeholders who can support our operations, whether through purchases or partnerships. Us being selected as an Innovation AveNEW company and winning the SLAS Ignite Award puts us at the right place to connect with the people who would most benefit from adopting SHS in preclinical research. We know there are people who want to make solubility measurements easier and use it to inform their decision making. Many of them attend automation conferences seeking ways to make tedious processes more humane and bearable in terms of workload.
We had a chance at the spotlight in SLAS Europe 2025, and made best use of it. We received full attendance and a free stand upon being selected, which would not be financially possible if we had to pay for attendance and a booth. Being able to enter platforms like SLAS Europe 2025 also helps us fight the conservatism rampant in the pharmaceutical industry. Our cache of application notes and collaboration showcases now has a place on an international platform. And that’s an opportunity I’m grateful to receive from SLAS.
Author
-

Paul Naphtali is a seasoned online marketing consultant. He brings to the table three years of online marketing and copywriting experience within the life sciences industry. His MSc and PhD experience also provides him with the acumen to understand complex literature and translate it to any audience. This way, he can fulfill his passion for sharing the beauty of biomedical research and inspiring action from his readers.
View all posts