The magic bullet of biomedicine
The magic bullet. You’ve probably heard it when your friends dubiously find the one thing that will solve all their problems.
The term itself comes from German folklore and the figure known as Der Freischütz. This figure is a marksman who obtains seven magic bullets. Six hit any object he pleases without fail. The last, however, is at the absolute servitude of the devil.
Thankfully, the magic bullet we seek in life science research does not have such a morbid use. Rather, biomedical professionals seek drugs that can treat patients without causing side effects and major burdens on patient well-being.
With such a huge life sciences industry, we’ve seen therapeutics from biomolecules like monoclonal antibodies, to small molecules as small as aspirin. Biomolecules can bind molecular targets with high selectivity but they need to be injected, limiting their use. Orally available small molecules are far more convenient and accessible to patients but they often have serious side effects.
But what if there was a kind of molecule that’s just right? One that retains the selectivity of proteins, but can penetrate cells and integrates the ease of producing, administering, and storing small molecules? Enter ThirdLaw Molecular, an Innovation AveNEW company for SLAS Europe 2025.
The company developed a new class of biomolecules called Spiroligomer™ molecules. These molecules use unnatural building blocks, similar to natural amino acids, to form systems of fused rings of any length. Spiroligomer™ molecules can also integrate any kind of side group to expand the kinds of target molecules that they bind. Such a strong modality allows researchers to develop new therapeutics and diagnostics for previously undruggable diseases, with increased affordability and accessibility.
Want to hear more? Then read along my interview with Christian Schafmeister, Founder and President of ThirdLaw Molecular. He’ll tell the story behind Spiroligomers, their many applications, and how he plans to accelerate drug development with his collaborators and at SLAS Europe 2025.
The interview
Introducing Spiroligomers
PN: Your company has developed a new class of compounds called Spiroligomer™ molecules. What are they?
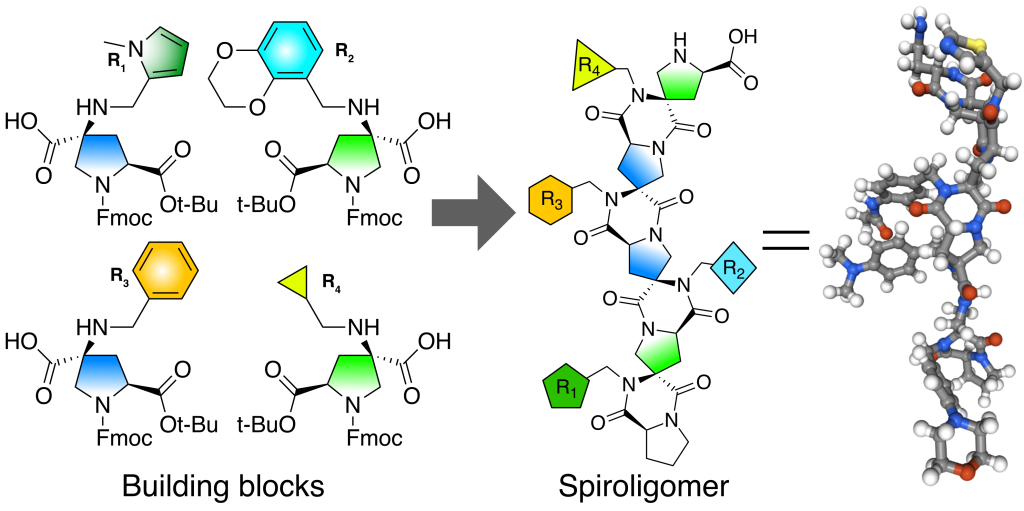
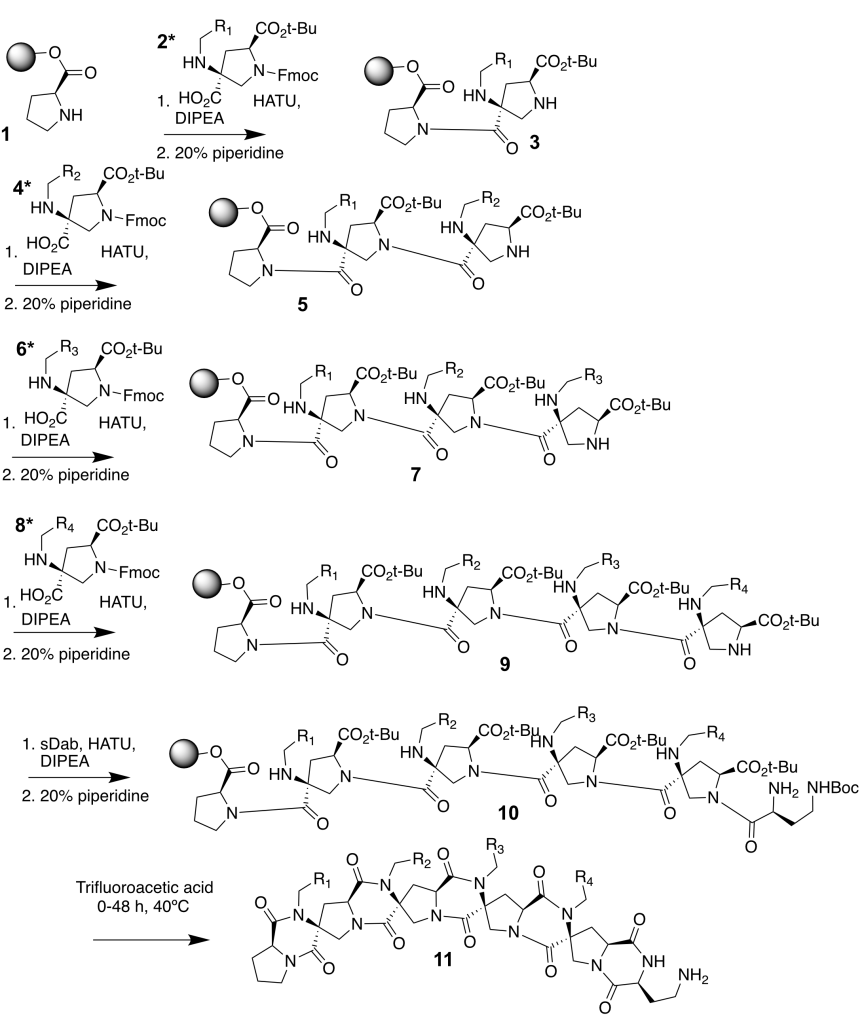
CS: Spiroligomer™ molecules comprise a new class of compounds of our creation (Figure 1). They have a backbone made of fused rings that are connected to form ladder-like molecules. These rings are built from a modified amino acid called hydroxyproline. This molecule may sound foreign, but it’s what makes the cartilage strong across your body. We took natural hydroxyproline and turned it into diverse new cyclic building blocks that have two amino acid groups on each of them. Pairs of amide bonds then connect the cyclic building blocks together. With this setup, we can form rings fused to rings and make them any length we want. These rings have carbon atoms within them called “embedded stereocenters”, these exist in two different three-dimensional arrangements that twist the fused-ring molecules into different shapes. In addition to this, the building blocks display diverse sidechains that change the outside of the molecule in controlled ways. Every feature of Spiroligomer molecules can be controlled to create molecules tailored to bind proteins selectively.
PN: How did you come up with these molecules?
CS: It’s always been my goal to create molecules that could do the things that proteins can. Proteins are the building blocks of life. They accelerate chemical reactions, bind all kinds of molecules, and can selectively transport things across membranes. I started out trying to build proteins but found them frustrating because they need to undergo a complex folding process to carry out their function and folding cannot be easily controlled. The very trait that enables their ubiquity also made protein design a stressful experience.
That got me wondering: how do I get rid of protein folding?
I started with the amino acids through which proteins are built. I linked them in different ways. I tried multiple bonds, rotating the amino acids, and forming rings too. My efforts converged into building new building blocks and connecting them through fused ring systems, or ladders. This system let me control what rings I could put into each position and how they’re oriented, forming unique structures with complex shapes.
That’s how I made Spiroligomer™ molecules.
PN: When you came up with Spiroligomer™ molecules, how did you subsequently come up with the company name, ThirdLaw Molecular?
CS: The most common active molecules in nature are multiple fused rings, like steroids (Figure 2). However, steroid synthesis takes on multiple time-consuming steps. With Spiroligomer™ molecules, we can scale up the synthesis of fused ring structures and use them for all kinds of applications. As we advance this technology, it’s my hope that its ability to support preclinical research becomes indistinguishable from magic. I took inspiration of this sentiment from the third law of science fiction by Arthur C. Clarke and decided to name the company, “ThirdLaw Molecular”.
SpiroligomerTM molecules at work
PN: I’m a huge bookworm, and I love how you conceived the name of your company. Now, I’d like to discuss how these molecules can make medical care more cost-effective and faster. What kind of magic can Spiroligomer™ molecules do?
CS: Proteins are the machinery of life. Their capabilities come from the ability of different sequences to fold into different conformations that have amazing activities. Over billions of years, each protein has mutated and maintained its ability to fold into their active shapes, however, at the same time, they are delicate and it is just as easy for proteins to unfold and lose their biologic activity. We’ve seen this problem manifest when using antibodies for diagnostic tests. Their short expiry dates mean that lots of antibody-based testing kits are discarded. Antibodies are also huge structures at over 150 kDa in size. The large size of antibodies make it impossible for them to enter cells and act as therapeutics within cells.
Spiroligomer™ molecules address both issues like so:
- They’re not too big, and not too small: Current drugs typically fall into one of two categories: small molecules such as aspirin (less than 500 Da) that can penetrate cells but only hit ~850 out of the 30,000 human proteins have been targeted. Small molecules have poor selectivity leading to off-target effects. On the other hand, biologics like antibodies (150 kDa) have much better selectivity and fewer side effects, but are limited to extracellular targets and have poor bioavailability and so they require administration by injection. . Until now, there have been very few drugs in the middle-sized range because it’s hard to make compounds whose shape we can control at that size range. With Spiroligomer™ molecules, we have one of the only ways of making that possible, effectively combining the precision of biologics with the desirable drug properties (cell penetration and oral availability) of small molecules, opening up the potential to target undruggable proteins
- They are rigid but retain versatility: Spiroligomer™ molecules are structured so that many side chains can hang off their core ladder structure. With an almost infinite number of possible combinations of side groups, we can prepare huge libraries and assay them to find those Spiroligomer™ molecules that will bind proteins we are targeting.
PN: Have you already tested Spiroligomer™ molecules for various applications?
CS: I can name one major application to start. We’re developing a number of Spiroligomer™ molecules that target tumor necrosis factor-a (TNF-a). You’ve probably heard of Himura at some point. It’s an antibody 150 kDA in size that treats rheumatoid arthritis (RA) by targeting TNF-a. Because it’s so big, you can’t swallow it to receive its benefits; you have to inject it intravenously. In contrast, our technology has the potential for a patient to reduce RA symptoms just by taking a Spiroligomer™ pill instead of a much more difficult to administer intravenous injection.
Spiroligomer™ molecules also have massive potential for diagnostic use. Back in 2018, I pitched the possibility of developing Spiroligomer™ molecules that bind to proteins and take part in lateral flow assays (LFAs). You’ll have been well-acquainted with LFAs; they were critical for limiting COVID-19 spread by providing at home diagnostic tests for identifying SARS-CoV-2 positive cases. The Department of Defense believed their potential when we pitched the idea in 2018. They then commissioned us to develop diagnostic tests for emerging threats as part of their rapid response plan.
The future of SpiroligomerTM Molecules
PN: With so many potential directions to take Spiroligomer™ molecules, what kind of a development trajectory are you hoping for your class of molecules?
CS: Our hope is that we can license the technology as widely as possible. The sheer number of molecules we can develop into drugs confers it with unlimited potential for treating any disease.
The potential for Spiroligomer™ molecules in the clinic leaves us eager to set up partnerships with companies big and small. Anyone who seeks to target a protein for disease can engage us to screen libraries for compounds that binds to their protein of interest. Together, we can develop drugs to improve how we diagnose, manage, and treat disease.
PN: SLAS Europe 2025 is all about automation. Have you considered making Spiroligomer™ molecules easy to develop for automated workflows given the huge potential for high-throughput drug development?
CS: Absolutely. Spiroligomer™ molecules enable the development of software that can design starting points for therapeutic development from known crystal structures of target proteins. You can build them with any combination of stereochemistry in the backbone and side chains. They’re as easy to build together as bricks from a LEGO set.
The best part is that we can use existing technologies to assemble Spiroligomer™ molecules. For one, we have developed the technology to easily assemble them on robots. You can also attach Spiroligomer™ molecules to DNA as part of a DNA-encoded library. These would help you determine which building blocks were used for a given drug candidate, as well as which ones bound most effectively to a protein target.
With such potential for automation, we’ve made it much easier for companies to integrate Spiroligomer™ technology into drug development workflows.
PN: I can see now that your selection for SLAS Europe 2025 was well-deserved. Congratulations on this awesome achievement! What are you hoping to do while you’re at the event, and where will you take your Spiroligomer™ technology next?
CS: The pharmaceutical industry is conservative. In many ways, that’s a good thing; we want to know that we are administering safe therapies to our patients. Being averse to risk also comes with greater hurdles for companies like ours offering new solutions to life-changing diseases that ail us. Despite the promise of Spiroligomer™ molecules, we still need to conduct more testing to prove their safety as well as their efficacy. Because of that, we’re currently raising money to further demonstrate that our molecules can be safe and effective drugs.
Becoming one of the companies selected as an Innovation AveNEW company for SLAS Europe 2025 will help us immensely towards that goal. The conference will give us opportunities to establish partnerships with other companies engaged in preclinical development. Similar partnerships with automation companies can also help us make our molecules more compatible with robotics and other automated systems. With a huge network at our disposal, we’re excited to see what we learn at SLAS Europe 2025 and how we can form new collaborations by being an Innovation AveNEW company.
Author
-

Paul Naphtali is a seasoned online marketing consultant. He brings to the table three years of online marketing and copywriting experience within the life sciences industry. His MSc and PhD experience also provides him with the acumen to understand complex literature and translate it to any audience. This way, he can fulfill his passion for sharing the beauty of biomedical research and inspiring action from his readers.
View all posts